| Line 6: | Line 6: | ||
Shechtman’s discovery was extremely controversial because everyone supposedly knew that his discovery was against the laws of nature. For this reason, he waited two years to publish his results, but he received the Nobel Prize in Chemistry in 2011 for his “impossible” findings[https://www.goldennumber.net/quasi-crystals/]. Shechtman’s discoveries were named “quasicrystals” due to their unique nature (quasi- means seemingly, but not really). | Shechtman’s discovery was extremely controversial because everyone supposedly knew that his discovery was against the laws of nature. For this reason, he waited two years to publish his results, but he received the Nobel Prize in Chemistry in 2011 for his “impossible” findings[https://www.goldennumber.net/quasi-crystals/]. Shechtman’s discoveries were named “quasicrystals” due to their unique nature (quasi- means seemingly, but not really). | ||
| + | |||
| + | <center> | ||
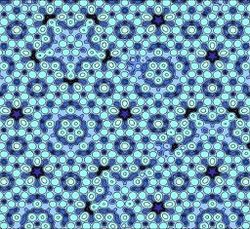
| + | [[File:Atomic model quasicrystal.jpg|250px|An atomic model of a quasicrystal]] <br> | ||
| + | <small> [https://www.nytimes.com/2011/10/06/science/06nobel.html The New York Times] </small> | ||
| + | </center> | ||
The Nobel Prize demonstrated a paradigm shift in the way chemists viewed solid matter and crystals. Scientists started to look for aperiodic and Penrose tiling around them that were similar in structure to the quasicrystals, and they found them in the Islamic mosaics (discussed in the [[MA271Fall2020Walther_Topic27_Real World Examples|Real World Examples]] section) in Spain and Iran. | The Nobel Prize demonstrated a paradigm shift in the way chemists viewed solid matter and crystals. Scientists started to look for aperiodic and Penrose tiling around them that were similar in structure to the quasicrystals, and they found them in the Islamic mosaics (discussed in the [[MA271Fall2020Walther_Topic27_Real World Examples|Real World Examples]] section) in Spain and Iran. | ||
Electron diffraction confirms this strange quasicrystal phenomenon (now called quasiperiodicity) and shows symmetry orders such as five-fold that are unusual in most natural crystals and are more akin to the prototiles’ five-fold symmetry in Penrose tiling. This proves that 3D solids can be formed with five-fold symmetry using a [[Walther_MA271_Fall2020_topic20|Fourier transform]] of a Penrose tiling, first conducted by crystallographer Alan_Lindsay_Mackay. | Electron diffraction confirms this strange quasicrystal phenomenon (now called quasiperiodicity) and shows symmetry orders such as five-fold that are unusual in most natural crystals and are more akin to the prototiles’ five-fold symmetry in Penrose tiling. This proves that 3D solids can be formed with five-fold symmetry using a [[Walther_MA271_Fall2020_topic20|Fourier transform]] of a Penrose tiling, first conducted by crystallographer Alan_Lindsay_Mackay. | ||
| + | |||
| + | <center> | ||
| + | [[File:Electron diffraction quasicrystal.jpg|250px|An electron diffraction pattern of a quasicrystal]] <br> | ||
| + | <small> [https://en.wikipedia.org/wiki/Quasicrystal#History Wikipedia] </small> | ||
| + | </center> | ||
As discussed previously, Penrose tiling are created by filling a 2D area with prototiles that 1) follow five-fold symmetry and 2) are proportioned by the golden ratio <math> \phi </math>. Before the discovery of quasicrystals, scientists and mathematicians alike thought that doing the same in 3D was impossible. Quasicrystals proved that ''solids'' could be created with ten-fold symmetry and still contain the same properties as Penrose tiling, except in 3D. Even more shocking is the fact that these 3D shapes can be created using one prototile, as opposed to the 2D Penrose tiling, which requires at least two prototiles to create. | As discussed previously, Penrose tiling are created by filling a 2D area with prototiles that 1) follow five-fold symmetry and 2) are proportioned by the golden ratio <math> \phi </math>. Before the discovery of quasicrystals, scientists and mathematicians alike thought that doing the same in 3D was impossible. Quasicrystals proved that ''solids'' could be created with ten-fold symmetry and still contain the same properties as Penrose tiling, except in 3D. Even more shocking is the fact that these 3D shapes can be created using one prototile, as opposed to the 2D Penrose tiling, which requires at least two prototiles to create. | ||
| + | |||
| + | <center> | ||
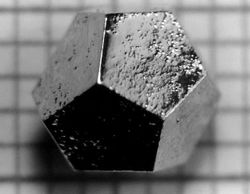
| + | [[File:Quasicrystal.jpg|250px|A quasicrystal as a solid]] <br> | ||
| + | <small> [http://www.geologyin.com/2018/01/what-are-quasicrystals-and-what-makes.html Geologyin] </small> | ||
| + | </center> | ||
At first, it was thought that the quasicrystals were not stable enough to be naturally occurring and could only be made in a lab, but then two naturally occurring quasicrystals were discovered in a meteorite recovered from Russia and in a Russian river, in 2009 and 2015 respectively[https://www.goldennumber.net/quasi-crystals/]. Now, quasicrystals are used in non-stick coatings in cooking appliances, as well as in developing stronger steel for armor and medical purposes. | At first, it was thought that the quasicrystals were not stable enough to be naturally occurring and could only be made in a lab, but then two naturally occurring quasicrystals were discovered in a meteorite recovered from Russia and in a Russian river, in 2009 and 2015 respectively[https://www.goldennumber.net/quasi-crystals/]. Now, quasicrystals are used in non-stick coatings in cooking appliances, as well as in developing stronger steel for armor and medical purposes. | ||
Revision as of 21:59, 6 December 2020
Quasicrystals
Penrose tiling have been seen in nature as well. In the field of biochemistry, scientists had originally thought crystals found in natural settings (not synthetically created by humans) are always symmetrical, which means they must be strictly periodic.
However, in 1982, scientist David Shechtman found in his electron microscope an image of the crystal he was studying (an aluminum-manganese alloy) that consisted of ten-fold symmetry. This was highly unusual, because most natural crystals contain two-, three-, four-, or six-fold symmetries, and that is what scientists believed was what defined a crystal in the first place.
Shechtman’s discovery was extremely controversial because everyone supposedly knew that his discovery was against the laws of nature. For this reason, he waited two years to publish his results, but he received the Nobel Prize in Chemistry in 2011 for his “impossible” findings[1]. Shechtman’s discoveries were named “quasicrystals” due to their unique nature (quasi- means seemingly, but not really).
The Nobel Prize demonstrated a paradigm shift in the way chemists viewed solid matter and crystals. Scientists started to look for aperiodic and Penrose tiling around them that were similar in structure to the quasicrystals, and they found them in the Islamic mosaics (discussed in the Real World Examples section) in Spain and Iran.
Electron diffraction confirms this strange quasicrystal phenomenon (now called quasiperiodicity) and shows symmetry orders such as five-fold that are unusual in most natural crystals and are more akin to the prototiles’ five-fold symmetry in Penrose tiling. This proves that 3D solids can be formed with five-fold symmetry using a Fourier transform of a Penrose tiling, first conducted by crystallographer Alan_Lindsay_Mackay.
As discussed previously, Penrose tiling are created by filling a 2D area with prototiles that 1) follow five-fold symmetry and 2) are proportioned by the golden ratio $ \phi $. Before the discovery of quasicrystals, scientists and mathematicians alike thought that doing the same in 3D was impossible. Quasicrystals proved that solids could be created with ten-fold symmetry and still contain the same properties as Penrose tiling, except in 3D. Even more shocking is the fact that these 3D shapes can be created using one prototile, as opposed to the 2D Penrose tiling, which requires at least two prototiles to create.
At first, it was thought that the quasicrystals were not stable enough to be naturally occurring and could only be made in a lab, but then two naturally occurring quasicrystals were discovered in a meteorite recovered from Russia and in a Russian river, in 2009 and 2015 respectively[2]. Now, quasicrystals are used in non-stick coatings in cooking appliances, as well as in developing stronger steel for armor and medical purposes.
Further Reading:
- The Wikipedia page for quasicrystals is very technical and contains many esoteric explanations, so proceed with caution when reading it.
- https://www.vice.com/en/article/4x3me3/quasicrystals-are-natures-impossible-matter --> Here is an interesting article about how quasicrystals break our understanding of physics and their possible future.