| Line 41: | Line 41: | ||
== Particle-in-a-box == | == Particle-in-a-box == | ||
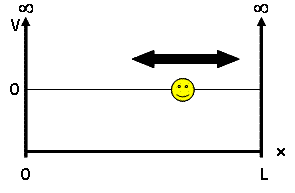
| − | For our discussion, we will be looking at the particle-in-a-box model - a system which defines a particle of mass <math> m </math> and potential energy <math> V(x) </math>. | + | For our discussion, we will be looking at the particle-in-a-box model - a system which defines a particle of mass <math> m </math> and potential energy <math> V(x) </math>. This mass is placed into a 'box', a 1D universe where potential energy is 0 inside the box but infinite outside, confining our particle to the inside of the box: |
| + | |||
| + | [[Image:ParticleInABox.gif]] | ||
Revision as of 13:13, 6 December 2020
Contents
Schrödinger Equation
Varun Chheda
Dr. Walther
Table of Contents
Introduction
The Wavefunction
Particle-in-a-box
Derivation
The Hydrogen Atom
Quantum Tunneling
References and Further Readings
Introduction
First postulated in 1925 by its namesake discoverer, Erwin Schrodinger, the Schrodinger equation is used to describe the wavefunction of particles – that is, an equation that unifies the wave and particle nature of the energy of matter. In its most simple form, it is expressed as a partial differential equation between its energy, representing the wave characteristic, and the Hamiltonian, describing its particle nature. The equation became the foundation for the field known today as quantum mechanics. Although expressible in many different forms, in this paper we limit our scope to perhaps the most famous and simple systems, the particle-in-a-box. We will then examine applications of the Schrodinger equation in modeling of the hydrogen atom and quantum tunneling.
The Wavefunction
The wavefunction In order to begin our exploration of the wonder of the Schrodinger equation, we must begin, (as always?), with definition. Central to the Schrodinger equation is the idea of a wavefunction, an equation which, very simply, takes on values based upon the position of the particle in question. Prior to Schrodinger's formulation, physicists had no way of describing both the particle and wave nature of particles in a single equation. Schrodinger's insight was to use the wavefunction as a wave analog to the precise momentum and directions of particles (after all, such terms are meaningless in waves). For our explicit investigation (and my sanity/calculus knowledge) we will limit our calculation to the 1D particle-in-a-box model, but we will discuss radial and angular wavefunctions as applied in hydrogenlike wavefunctions stemming from the Bohr model.
We use the Greek letter $ \psi $ to represent a wavefunction, but what does it mean? Physically, the Born interpretation of the wavefunction states the probability of finding a particle in a given region is proportional to the value of the wavefunction $ \psi $ squared. Indeed, $ \psi^2 $ is given its own definition as a probability density, which we have encountered before - the probability of finding the particle in the region divided by the region's volume.
A final note: Although $ \psi $ can take on negative values, (importantly) $ \psi^2 $ cannot - a result we will require later.
Particle-in-a-box
For our discussion, we will be looking at the particle-in-a-box model - a system which defines a particle of mass $ m $ and potential energy $ V(x) $. This mass is placed into a 'box', a 1D universe where potential energy is 0 inside the box but infinite outside, confining our particle to the inside of the box: