| (34 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:MA453Fall2013Walther]] | [[Category:MA453Fall2013Walther]] | ||
| − | == Crystals and | + | == Crystals and Symmetry == |
| − | '''Names | + | '''Names''' <br><hr> |
Jason Krupp (krupp@purdue.edu) <br> | Jason Krupp (krupp@purdue.edu) <br> | ||
Erik Plesha (eplesha@purdue.edu) <br> | Erik Plesha (eplesha@purdue.edu) <br> | ||
Andrew Wightman (awightma@purdue.edu) <br> | Andrew Wightman (awightma@purdue.edu) <br> | ||
| − | + | <br> | |
| − | + | '''Project Outline''' <br><hr> | |
| − | '''Project Outline | + | A) Crystal Symmetries Introduction<br> |
| − | A) Crystal Symmetries | + | *Miller Indices<br> |
| − | + | *Crystal Structures<br> | |
| − | + | *Slip Systems<br> | |
| − | + | ||
B) Crystal Movement and Symmetry<br> | B) Crystal Movement and Symmetry<br> | ||
| − | + | *Translational Movement<br> | |
| − | + | *Rotational Movement<br> | |
| − | + | *Mirror Movement<br> | |
| − | C)Combinations of Symmetry Operations<br> | + | C) Combinations of Symmetry Operations<br> |
| − | + | *32 Crystal Classes<br> | |
| − | D)Crystal Symmetry Groups<br> | + | D) Crystal Symmetry Groups<br> |
| − | + | *Finite Symmetry Groups<br> | |
| − | -- | + | *Non-Finite Symmetry Groups<br> |
| − | <br><br> | + | <br> |
| − | '''References | + | '''Crystal Symmetries Introduction'''<br><hr><br> |
| − | Gallian, J. (2013). Contemporary abstract algebra. (8th ed.). Boston, MA: Brooks/Cole, Cengage Learning. | + | Many important material properties depend on crystal structure. Some of these <br> |
| + | include the following inexhaustive list: conductivity, magnetism, stiffness, and<br> | ||
| + | strength. <br> | ||
| + | Miller Indices represent an efficient way to label the orientation of the crystals.<br> | ||
| + | For planes, the Miller Index value is the reciprocal of the value of the <br> | ||
| + | intersection of the plane with a particular axis, converted to whole numbers and are <br> | ||
| + | usually represented by round brackets (parenthesis). For directions in a crystal <br> | ||
| + | lattice, the index is the axis coordinate of the end point of the vector, converted <br> | ||
| + | to the nearest whole number and are usually represented by [square brackets]. <br> | ||
| + | |||
| + | [[Image:Miller.PNG]] <br> | ||
| + | |||
| + | For example, the figure above depicts 3 of the 6 cube faces and the corresponding <br> | ||
| + | Miller Indices. The red plane is labeled as (100) because the plane is shifted 1 <br> | ||
| + | unit in the x-direction. The yellow plane is labeled (010) because it is shifted 1 <br> | ||
| + | unit in the y-direction. Finally, the green plane is labeled (001) because it is <br> | ||
| + | shifted 1 unit in the z-direction. For more on Miller Indices, please visit the <br> | ||
| + | link listed in the References Section.<br> | ||
| + | Although Miller Indices do a great job of describing crystals, it doesn't complete <br> | ||
| + | the task. Crystals can also be divided up according to their structure, the three most <br> | ||
| + | common types being FCC (Face-Centered Cubic), BCC (Body-Centered Cubic), and SC <br> | ||
| + | (Simple Cubic) structures. <br> | ||
| + | |||
| + | [[Image:crystal types.PNG]] <br> | ||
| + | |||
| + | As you can see, the above figure shows the three aforementioned crystal types. The <br> | ||
| + | body-centered cubic structure is similar to the simple cubic structure but with an added <br> | ||
| + | atom in the center of the unit cell. The face-centered cubic structure is also similar <br> | ||
| + | to the simple cubic structure but with added atoms in the center of all 6 cube faces. <br> | ||
| + | These structures can also be defined by their coordination numbers, or the number of <br> | ||
| + | nearest neighboring atoms. For the simple cubic structure, there are 6 nearest neighbors, <br> | ||
| + | corresponding to a coordination number of 6. The face-centered cubic structure has a <br> | ||
| + | coordination number of 12 and the body-centered cubic structure has a coordination number <br> | ||
| + | of 8. <br> | ||
| + | |||
| + | A slip system is a combination of a slip direction and a slip plane. A slip plane is a plane <br> | ||
| + | in which the planar density is largest. For the FCC crystal structure, the slip plane is <br> | ||
| + | labeled (111) because this plane is most densely populated with atoms and has the smallest <br> | ||
| + | amount of free space. The slip direction is the most densely populated direction of a crystal; <br> | ||
| + | this is the [110] direction for FCC crystals. With increasing load to the crystal, the slip <br> | ||
| + | plane and direction align parallel to the tensile stress axis. Under extreme tension, crystal <br> | ||
| + | fracture may be observed. | ||
| + | |||
| + | |||
| + | '''Combinations of Symmetry Operations'''<br><hr><br> | ||
| + | Symmetry within a crystal is a systematic repetition of the structural features of the crystal. <br> | ||
| + | There are two main types of symmetry that exist within a crystal system. The first of the symmetries <br> | ||
| + | is translational symmetry, which is the symmetry across a length or an area/volume. The other type of <br> | ||
| + | symmetry is point symmetry, which is the repetition of something around a point. There are four different <br> | ||
| + | types of point symmetry operations, reflection, rotation, inversion, and rotoinversion. Reflection is when <br> | ||
| + | one side of the crystal matches the other side of the crystal across a plane. A rotation occurs when the <br> | ||
| + | crystal is rotated a certain amount of degrees before it repeats itself. An inversion occurs when a line <br> | ||
| + | is drawn through the center and matches up with another feature of the crystal. A rotoinversion is when <br> | ||
| + | a rotation is performed with an inversion. <br><br> | ||
| + | When looking at all the different symmetry operations that a crystal structure can possess, crystals can <br> | ||
| + | be grouped into several different crystal systems. There are six main crystal systems that a crystal can <br> | ||
| + | belong in. Each crystal system possesses a certain symmetry operations that distinguish the system from <br> | ||
| + | the others. The six main crystal systems are triclinic system, monoclinic system, orthorhombic system, <br> | ||
| + | tetragonal system, hexagonal system, and isometric system. Within the six main crystal system exist <br> | ||
| + | crystal classes, each with their own combination of symmetry operations. In total, there are found to <br> | ||
| + | be a total of 32 crystal classes within the crystal systems. <br><br> | ||
| + | |||
| + | The first of the crystal systems that will be talked about is the triclinic system. The triclinic system <br> | ||
| + | is characterized by having a single 1-fold or 1-fold rotoinversion axis. This means that a crystal within <br> | ||
| + | the triclinic system can have no symmetry at all. The two different classes within the triclinic system are <br> | ||
| + | the pedial class and the pinacoidal class. The pedial class has no symmetry at all. The pinacoidal class has <br> | ||
| + | a center of symmetry, i. The image below shows a typical lattice for the triclinic crystal system. <br> | ||
| + | [[Image: triclinic.PNG]] <br><br> | ||
| + | |||
| + | The next of the crystal systems is the monoclinic system. The monoclinic system is characterized by mirror <br> | ||
| + | planes or a single 2-fold axis. The three crystal classes that sit within this crystal system are the <br> | ||
| + | sphenoidal class, the domatic class, and the prismatic class. The sphenoidal class is characterized by <br> | ||
| + | a 1A_2 symmetry and a single 2-fold rotational axis. The domatic class is characterized by a single mirror <br> | ||
| + | plane the prismatic class is characterized by a 2 fold axis and a single mirror axis. Below are images of the <br> | ||
| + | typical lattice structure for the monoclinic crystal system. The first is simple lattice and the other is base <br> | ||
| + | centered. <br> | ||
| + | [[Image:monoclinic basic.PNG]] [[Image:monoclinic base centered.PNG]]<br><br> | ||
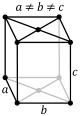
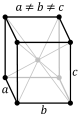
| + | The third of the crystal systems is the orthorhombic system. This system is characterized by a two fold axis or a <br> | ||
| + | two fold axis and two mirror planes. The crystal classes that sit within this system are rhombic-disphenoidal, <br> | ||
| + | rhombic-pyramidal, and rhombic-dipyramidal. The rhombic-disphenoidal is characterized by 3-two fold axis that are <br> | ||
| + | perpendicular to each other. The rhombic-pyramidal is characterized by 1 two fold rotational axis and 2 mirror planes. <br> | ||
| + | It also has 4 identical faces. The rhombic-dipyramidal has 3 2-fold axis and 3-mirror planes. There are four identical faces <br> | ||
| + | on the top and 4 identical faces on the bottom. The four images below show the lattice structures for the orthorhombic system.<br> | ||
| + | The images are simple, base centered, body centered, and face centered respectively. <br> | ||
| + | [[Image:ortho simple.PNG]][[Image:ortho base.PNG]][[Image:ortho body.PNG]][[Image:ortho face.PNG]] <br><br> | ||
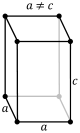
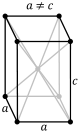
| + | The next crystal system is the tetragonal system. The tetragonal system is characterized by single 4-fold axis or <br> | ||
| + | a 4-fold rotoinversion axis. The Classes that are within the tetragonal system are the tetragonal-pyramidal class, <br> | ||
| + | the tetragonal-disphenoidal class, the tetragonal-dipyramidal class, the tetragonal-trapezohedral class, the <br> | ||
| + | ditetragonal-pyramid class, the tetragonal-scalenohedral class, and the ditetragonal-dipyrimidal class. Each of the <br> | ||
| + | classes listed have a combination of what defines a crystal to be within the tetragonal system. The image below shows <br> | ||
| + | the simple and body-centered lattice for the tetragonal system. <br> | ||
| + | [[Image:tetra simple.PNG]][[Image:tetra body.PNG]]<br><br> | ||
| + | The next crystal sustem is the hexagonal system. This system has no 4-fold axis, but it has at least 1 6-fold axis or <br> | ||
| + | 3-fold axis. The hexagonal system is the largest of all the systems consisting of 12 different crystal classes. <br> | ||
| + | The twelve different classes that are present within the hexagonal system are trigonal-pyramidal, rhombohedral, <br> | ||
| + | trigonal-trapezohedral, ditrigonal-pyramidal, hexagonal-scalenohedral, hexagonal-pyramidal, trigonal-dipyramidal, <br> | ||
| + | hexagonal-dipyramidal, hexagonal-trapezohedral, dihexagonal-pyramidal, ditrigonal-dipyramidal, and dihexagonal-dipyramidal.<br> | ||
| + | Each of the classes possess a symmetry that classifies the hexagonal crystal system. The image below shows the lattice <br> | ||
| + | structure for the hexagonal crystal system. <br> | ||
| + | [[Image:Hexagonal.PNG]]<br><br> | ||
| + | |||
| + | The last of the crystal systems is the isometric crystal system. This can also be referred to as the cubic system. <br> | ||
| + | The system is characterized by 4 3-fold axis or 4 3-fold rotoinversion axis. There are a total of five different <br> | ||
| + | within this crystal system. The classes are tetaroidal, diploidal, gyroidal, hextetrahedral, and hexoctahedral. <br> | ||
| + | The five crystal classes possess the characteristics of the isometric crystal system. The below images show the <br> | ||
| + | simple, body centered, and face centered lattice of the isometric crystal system.<br> | ||
| + | [[Image:cube simple.PNG]][[Image:cube body.PNG]][[Image:cube face.PNG]]<br><br> | ||
| + | |||
| + | By analyzing the symmetries of different crystals, one can categorize the crystal within one of the 32 crystal <br> | ||
| + | classes from above. | ||
| + | |||
| + | |||
| + | '''Crystal Symmetry Groups'''<br><hr><br> | ||
| + | |||
| + | As mentioned before there are 32 Crystal Classes corresponding to 7 different crystal systems or families. Each of the 32 <br> | ||
| + | crystal classes can be identified with its own symmetry group, however, many crystal structures have a different point symmetry. <br> | ||
| + | So, many crystals may have the same symmetric group structure, but can be further differentiated by their point symmetry. <br> | ||
| + | |||
| + | {| border="1" | ||
| + | |- | ||
| + | |'''Crystal System''' || '''Crystal Class''' || '''Point Symmetry''' || '''Order''' || '''Symmetry Group''' | ||
| + | |- | ||
| + | | rowspan=2| triclinic | ||
| + | | pedial | ||
| + | | enantiomorphic polar | ||
| + | | 1 | ||
| + | | <math>\mathbb{Z}_1</math> | ||
| + | |- | ||
| + | | pinacodoidal | ||
| + | | centrosymmetric | ||
| + | | 2 | ||
| + | | <math>\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | rowspan=3| monoclinic | ||
| + | | sphenoidal | ||
| + | | enantiomorphic polar | ||
| + | | 2 | ||
| + | | <math>\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | domatic | ||
| + | | polar | ||
| + | | 2 | ||
| + | | <math>\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | prismatic | ||
| + | | centrosymmetric | ||
| + | | 4 | ||
| + | | <math>\mathbb{V}</math> | ||
| + | |- | ||
| + | | rowspan=3| orthorhombic | ||
| + | | sphenoidal | ||
| + | | enantiomorphic | ||
| + | | 4 | ||
| + | | <math>\mathbb{V}</math> | ||
| + | |- | ||
| + | | pyramidal | ||
| + | | polar | ||
| + | | 4 | ||
| + | | <math>\mathbb{V}</math> | ||
| + | |- | ||
| + | | bipyramidal | ||
| + | | centrosymmetric | ||
| + | | 8 | ||
| + | | <math>\mathbb{V}\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | rowspan=7| tetragonal | ||
| + | | pyramidal | ||
| + | | enanitomorphic polar | ||
| + | | 4 | ||
| + | | <math>\mathbb{Z}_4</math> | ||
| + | |- | ||
| + | | disphenoidal | ||
| + | | non-centrosymmetric | ||
| + | | 4 | ||
| + | | <math>\mathbb{Z}_4</math> | ||
| + | |- | ||
| + | | dipyramidal | ||
| + | | centrosymmetric | ||
| + | | 8 | ||
| + | | <math>\mathbb{Z}_4\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | trapezoidal | ||
| + | | enantiomorphic | ||
| + | | 8 | ||
| + | | <math>\mathbb{D}_8</math> | ||
| + | |- | ||
| + | | ditetragonal-pyramidal | ||
| + | | polar | ||
| + | | 8 | ||
| + | | <math>\mathbb{D}_8</math> | ||
| + | |- | ||
| + | | scalenoidal | ||
| + | | non-centrosymmetric | ||
| + | | 8 | ||
| + | | <math>\mathbb{D}_8</math> | ||
| + | |- | ||
| + | | ditetragonal-dipyramidal | ||
| + | | centrosymmetric | ||
| + | | 16 | ||
| + | | <math>\mathbb{D}_8\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | rowspan=5| trigonal | ||
| + | | pyramidal | ||
| + | | enantiomorphic polar | ||
| + | | 3 | ||
| + | | <math>\mathbb{Z}_3</math> | ||
| + | |- | ||
| + | | rhombohedral | ||
| + | | centrosymmetric | ||
| + | | 6 | ||
| + | | <math>\mathbb{Z}_6</math> | ||
| + | |- | ||
| + | | trapezoidal | ||
| + | | enantiomorphic | ||
| + | | 6 | ||
| + | | <math>\mathbb{D}_6</math> | ||
| + | |- | ||
| + | | ditrigonal-pyramidal | ||
| + | | polar | ||
| + | | 6 | ||
| + | | <math>\mathbb{D}_6</math> | ||
| + | |- | ||
| + | | ditrigonal-scalahedral | ||
| + | | centrosymmetric | ||
| + | | 12 | ||
| + | | <math>\mathbb{D}_{12}</math> | ||
| + | |- | ||
| + | | rowspan="7" | hexagonal | ||
| + | | pyramdial | ||
| + | | enantiomorphic polar | ||
| + | | 6 | ||
| + | | <math>\mathbb{Z}_6</math> | ||
| + | |- | ||
| + | | trigonal-dipyramidal | ||
| + | | non-centrosymmetric | ||
| + | | 6 | ||
| + | | <math>\mathbb{Z}_6</math> | ||
| + | |- | ||
| + | | dipyramidal | ||
| + | | centrosymmetric | ||
| + | | 12 | ||
| + | | <math>\mathbb{Z}_6\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | trapezoidal | ||
| + | | enantiomorphic | ||
| + | | 12 | ||
| + | | <math>\mathbb{D}_{12}</math> | ||
| + | |- | ||
| + | | dihexagonal_pyramidal | ||
| + | | polar | ||
| + | | 12 | ||
| + | | <math>\mathbb{D}_{12}</math> | ||
| + | |- | ||
| + | | ditrigonal-dipyramidal | ||
| + | | non-centrosymmetric | ||
| + | | 12 | ||
| + | | <math>\mathbb{D}_{12}</math> | ||
| + | |- | ||
| + | | dihexagonal-dipyramidal | ||
| + | | centrosymmetric | ||
| + | | 24 | ||
| + | | <math>\mathbb{D}_{12}\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | rowspan="5" | cubic | ||
| + | | terahedral | ||
| + | | enantiomorphic | ||
| + | | 12 | ||
| + | | <math>\mathbb{A}_4</math> | ||
| + | |- | ||
| + | | hextertrahedral | ||
| + | | non-centrosymmetric | ||
| + | | 24 | ||
| + | | <math>\mathbb{S}_4</math> | ||
| + | |- | ||
| + | | diploidal | ||
| + | | centrosymmetric | ||
| + | | 24 | ||
| + | | <math>\mathbb{A}_4\times\mathbb{Z}_2</math> | ||
| + | |- | ||
| + | | gyroidal | ||
| + | | enantiomorphic | ||
| + | | 24 | ||
| + | | <math>\mathbb{S}_4</math> | ||
| + | |- | ||
| + | | hexoctahedral | ||
| + | | centrosymmetric | ||
| + | | 48 | ||
| + | | <math>\mathbb{S}_4\times\mathbb{Z}_2</math> | ||
| + | |} | ||
| + | |||
| + | '''References and Links'''<br><hr> | ||
| + | Gallian, J. (2013). Contemporary abstract algebra. (8th ed.). Boston, MA: Brooks/Cole, Cengage Learning. <br> | ||
| + | |||
| + | [http://www.chem.qmul.ac.uk/surfaces/scc/scat1_1b.htm Miller Indices Link] <br> | ||
| + | |||
| + | [http://www.chem.ufl.edu/~itl/2045/lectures/lec_h.html Cubic Structures Link] <br> | ||
| + | |||
| + | Jacobson, L. A. (2008). Crystal symmetries - physical metallurgy. Unpublished manuscript, METE 327, Retrieved from <br> http://infohost.nmt.edu/~ljacobso/crystalslides.pdf <br> | ||
| + | |||
| + | [http://ocw.mit.edu/courses/materials-science-and-engineering/3-40j-physical-metallurgy-fall-2009/lecture-notes/MIT3_40JF09_lec06.pdf Slip Systems Reference] | ||
'''MA 453 Notes'''<hr> | '''MA 453 Notes'''<hr> | ||
| + | |||
| + | Nelson, S. A. (August 2013). External Symmetry of Crystals, 32 Crystal Classes. http://www.tulane.edu/~sanelson/eens211/32crystalclass.htm. <br> | ||
| + | |||
| + | Wikipedia: The Free Encyclopedia (November 15, 2013). Crystal Systems. http://en.wikipedia.org/wiki/Crystal_system. <br> | ||
Latest revision as of 08:11, 3 December 2013
Crystals and Symmetry
NamesJason Krupp (krupp@purdue.edu)
Erik Plesha (eplesha@purdue.edu)
Andrew Wightman (awightma@purdue.edu)
A) Crystal Symmetries Introduction
- Miller Indices
- Crystal Structures
- Slip Systems
B) Crystal Movement and Symmetry
- Translational Movement
- Rotational Movement
- Mirror Movement
C) Combinations of Symmetry Operations
- 32 Crystal Classes
D) Crystal Symmetry Groups
- Finite Symmetry Groups
- Non-Finite Symmetry Groups
Many important material properties depend on crystal structure. Some of these
include the following inexhaustive list: conductivity, magnetism, stiffness, and
strength.
Miller Indices represent an efficient way to label the orientation of the crystals.
For planes, the Miller Index value is the reciprocal of the value of the
intersection of the plane with a particular axis, converted to whole numbers and are
usually represented by round brackets (parenthesis). For directions in a crystal
lattice, the index is the axis coordinate of the end point of the vector, converted
to the nearest whole number and are usually represented by [square brackets].
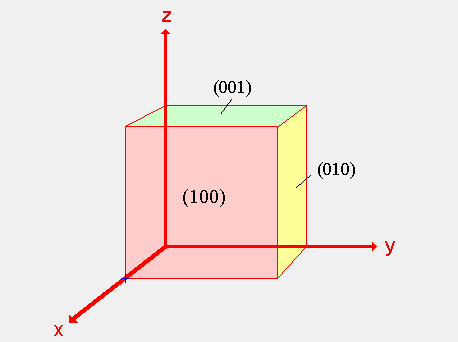
For example, the figure above depicts 3 of the 6 cube faces and the corresponding
Miller Indices. The red plane is labeled as (100) because the plane is shifted 1
unit in the x-direction. The yellow plane is labeled (010) because it is shifted 1
unit in the y-direction. Finally, the green plane is labeled (001) because it is
shifted 1 unit in the z-direction. For more on Miller Indices, please visit the
link listed in the References Section.
Although Miller Indices do a great job of describing crystals, it doesn't complete
the task. Crystals can also be divided up according to their structure, the three most
common types being FCC (Face-Centered Cubic), BCC (Body-Centered Cubic), and SC
(Simple Cubic) structures.
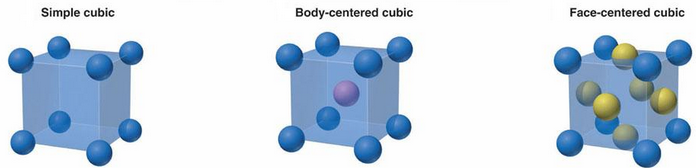
As you can see, the above figure shows the three aforementioned crystal types. The
body-centered cubic structure is similar to the simple cubic structure but with an added
atom in the center of the unit cell. The face-centered cubic structure is also similar
to the simple cubic structure but with added atoms in the center of all 6 cube faces.
These structures can also be defined by their coordination numbers, or the number of
nearest neighboring atoms. For the simple cubic structure, there are 6 nearest neighbors,
corresponding to a coordination number of 6. The face-centered cubic structure has a
coordination number of 12 and the body-centered cubic structure has a coordination number
of 8.
A slip system is a combination of a slip direction and a slip plane. A slip plane is a plane
in which the planar density is largest. For the FCC crystal structure, the slip plane is
labeled (111) because this plane is most densely populated with atoms and has the smallest
amount of free space. The slip direction is the most densely populated direction of a crystal;
this is the [110] direction for FCC crystals. With increasing load to the crystal, the slip
plane and direction align parallel to the tensile stress axis. Under extreme tension, crystal
fracture may be observed.
Symmetry within a crystal is a systematic repetition of the structural features of the crystal.
There are two main types of symmetry that exist within a crystal system. The first of the symmetries
is translational symmetry, which is the symmetry across a length or an area/volume. The other type of
symmetry is point symmetry, which is the repetition of something around a point. There are four different
types of point symmetry operations, reflection, rotation, inversion, and rotoinversion. Reflection is when
one side of the crystal matches the other side of the crystal across a plane. A rotation occurs when the
crystal is rotated a certain amount of degrees before it repeats itself. An inversion occurs when a line
is drawn through the center and matches up with another feature of the crystal. A rotoinversion is when
a rotation is performed with an inversion.
When looking at all the different symmetry operations that a crystal structure can possess, crystals can
be grouped into several different crystal systems. There are six main crystal systems that a crystal can
belong in. Each crystal system possesses a certain symmetry operations that distinguish the system from
the others. The six main crystal systems are triclinic system, monoclinic system, orthorhombic system,
tetragonal system, hexagonal system, and isometric system. Within the six main crystal system exist
crystal classes, each with their own combination of symmetry operations. In total, there are found to
be a total of 32 crystal classes within the crystal systems.
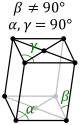
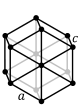
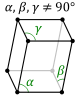
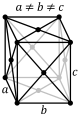
The first of the crystal systems that will be talked about is the triclinic system. The triclinic system
is characterized by having a single 1-fold or 1-fold rotoinversion axis. This means that a crystal within
the triclinic system can have no symmetry at all. The two different classes within the triclinic system are
the pedial class and the pinacoidal class. The pedial class has no symmetry at all. The pinacoidal class has
a center of symmetry, i. The image below shows a typical lattice for the triclinic crystal system.
The next of the crystal systems is the monoclinic system. The monoclinic system is characterized by mirror
planes or a single 2-fold axis. The three crystal classes that sit within this crystal system are the
sphenoidal class, the domatic class, and the prismatic class. The sphenoidal class is characterized by
a 1A_2 symmetry and a single 2-fold rotational axis. The domatic class is characterized by a single mirror
plane the prismatic class is characterized by a 2 fold axis and a single mirror axis. Below are images of the
typical lattice structure for the monoclinic crystal system. The first is simple lattice and the other is base
centered.
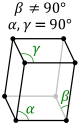
The third of the crystal systems is the orthorhombic system. This system is characterized by a two fold axis or a
two fold axis and two mirror planes. The crystal classes that sit within this system are rhombic-disphenoidal,
rhombic-pyramidal, and rhombic-dipyramidal. The rhombic-disphenoidal is characterized by 3-two fold axis that are
perpendicular to each other. The rhombic-pyramidal is characterized by 1 two fold rotational axis and 2 mirror planes.
It also has 4 identical faces. The rhombic-dipyramidal has 3 2-fold axis and 3-mirror planes. There are four identical faces
on the top and 4 identical faces on the bottom. The four images below show the lattice structures for the orthorhombic system.
The images are simple, base centered, body centered, and face centered respectively.
The next crystal system is the tetragonal system. The tetragonal system is characterized by single 4-fold axis or
a 4-fold rotoinversion axis. The Classes that are within the tetragonal system are the tetragonal-pyramidal class,
the tetragonal-disphenoidal class, the tetragonal-dipyramidal class, the tetragonal-trapezohedral class, the
ditetragonal-pyramid class, the tetragonal-scalenohedral class, and the ditetragonal-dipyrimidal class. Each of the
classes listed have a combination of what defines a crystal to be within the tetragonal system. The image below shows
the simple and body-centered lattice for the tetragonal system.
The next crystal sustem is the hexagonal system. This system has no 4-fold axis, but it has at least 1 6-fold axis or
3-fold axis. The hexagonal system is the largest of all the systems consisting of 12 different crystal classes.
The twelve different classes that are present within the hexagonal system are trigonal-pyramidal, rhombohedral,
trigonal-trapezohedral, ditrigonal-pyramidal, hexagonal-scalenohedral, hexagonal-pyramidal, trigonal-dipyramidal,
hexagonal-dipyramidal, hexagonal-trapezohedral, dihexagonal-pyramidal, ditrigonal-dipyramidal, and dihexagonal-dipyramidal.
Each of the classes possess a symmetry that classifies the hexagonal crystal system. The image below shows the lattice
structure for the hexagonal crystal system.
The last of the crystal systems is the isometric crystal system. This can also be referred to as the cubic system.
The system is characterized by 4 3-fold axis or 4 3-fold rotoinversion axis. There are a total of five different
within this crystal system. The classes are tetaroidal, diploidal, gyroidal, hextetrahedral, and hexoctahedral.
The five crystal classes possess the characteristics of the isometric crystal system. The below images show the
simple, body centered, and face centered lattice of the isometric crystal system.
By analyzing the symmetries of different crystals, one can categorize the crystal within one of the 32 crystal
classes from above.
As mentioned before there are 32 Crystal Classes corresponding to 7 different crystal systems or families. Each of the 32
crystal classes can be identified with its own symmetry group, however, many crystal structures have a different point symmetry.
So, many crystals may have the same symmetric group structure, but can be further differentiated by their point symmetry.
| Crystal System | Crystal Class | Point Symmetry | Order | Symmetry Group |
| triclinic | pedial | enantiomorphic polar | 1 | $ \mathbb{Z}_1 $ |
| pinacodoidal | centrosymmetric | 2 | $ \mathbb{Z}_2 $ | |
| monoclinic | sphenoidal | enantiomorphic polar | 2 | $ \mathbb{Z}_2 $ |
| domatic | polar | 2 | $ \mathbb{Z}_2 $ | |
| prismatic | centrosymmetric | 4 | $ \mathbb{V} $ | |
| orthorhombic | sphenoidal | enantiomorphic | 4 | $ \mathbb{V} $ |
| pyramidal | polar | 4 | $ \mathbb{V} $ | |
| bipyramidal | centrosymmetric | 8 | $ \mathbb{V}\times\mathbb{Z}_2 $ | |
| tetragonal | pyramidal | enanitomorphic polar | 4 | $ \mathbb{Z}_4 $ |
| disphenoidal | non-centrosymmetric | 4 | $ \mathbb{Z}_4 $ | |
| dipyramidal | centrosymmetric | 8 | $ \mathbb{Z}_4\times\mathbb{Z}_2 $ | |
| trapezoidal | enantiomorphic | 8 | $ \mathbb{D}_8 $ | |
| ditetragonal-pyramidal | polar | 8 | $ \mathbb{D}_8 $ | |
| scalenoidal | non-centrosymmetric | 8 | $ \mathbb{D}_8 $ | |
| ditetragonal-dipyramidal | centrosymmetric | 16 | $ \mathbb{D}_8\times\mathbb{Z}_2 $ | |
| trigonal | pyramidal | enantiomorphic polar | 3 | $ \mathbb{Z}_3 $ |
| rhombohedral | centrosymmetric | 6 | $ \mathbb{Z}_6 $ | |
| trapezoidal | enantiomorphic | 6 | $ \mathbb{D}_6 $ | |
| ditrigonal-pyramidal | polar | 6 | $ \mathbb{D}_6 $ | |
| ditrigonal-scalahedral | centrosymmetric | 12 | $ \mathbb{D}_{12} $ | |
| hexagonal | pyramdial | enantiomorphic polar | 6 | $ \mathbb{Z}_6 $ |
| trigonal-dipyramidal | non-centrosymmetric | 6 | $ \mathbb{Z}_6 $ | |
| dipyramidal | centrosymmetric | 12 | $ \mathbb{Z}_6\times\mathbb{Z}_2 $ | |
| trapezoidal | enantiomorphic | 12 | $ \mathbb{D}_{12} $ | |
| dihexagonal_pyramidal | polar | 12 | $ \mathbb{D}_{12} $ | |
| ditrigonal-dipyramidal | non-centrosymmetric | 12 | $ \mathbb{D}_{12} $ | |
| dihexagonal-dipyramidal | centrosymmetric | 24 | $ \mathbb{D}_{12}\times\mathbb{Z}_2 $ | |
| cubic | terahedral | enantiomorphic | 12 | $ \mathbb{A}_4 $ |
| hextertrahedral | non-centrosymmetric | 24 | $ \mathbb{S}_4 $ | |
| diploidal | centrosymmetric | 24 | $ \mathbb{A}_4\times\mathbb{Z}_2 $ | |
| gyroidal | enantiomorphic | 24 | $ \mathbb{S}_4 $ | |
| hexoctahedral | centrosymmetric | 48 | $ \mathbb{S}_4\times\mathbb{Z}_2 $ |
Gallian, J. (2013). Contemporary abstract algebra. (8th ed.). Boston, MA: Brooks/Cole, Cengage Learning.
Jacobson, L. A. (2008). Crystal symmetries - physical metallurgy. Unpublished manuscript, METE 327, Retrieved from
http://infohost.nmt.edu/~ljacobso/crystalslides.pdf
Nelson, S. A. (August 2013). External Symmetry of Crystals, 32 Crystal Classes. http://www.tulane.edu/~sanelson/eens211/32crystalclass.htm.
Wikipedia: The Free Encyclopedia (November 15, 2013). Crystal Systems. http://en.wikipedia.org/wiki/Crystal_system.